What is Molecular Geometry ?
Molecular Geometry is basically the three dimensional arrangement / shape / structure of atoms that form a molecule. When molecules are formed by chemical bond which means atoms bonding together, suborbitals involved in the bond or bonds create different molecular shapes depending on many factors.
For example, the water molecules are not linear, a water molecule is actually ‘V’ shaped and the angle formed between the two Hydrogen atoms and the Oxygen atom is approximately of 105° degrees.
molecular geometry of water molecule hydrogen oxygen
When we draw molecules in two dimensions, we most of the time think that these molecules are flat. But in fact they exist in several different shapes and forms.
The Chemical Composition and the Molecular Geometry of a molecule is what mainly determine the properties of the molecule. Such as taste, boiling point, magnetism, dynamic, polarity, color, and all other properties.
Molecular Geometry and different types of molecular structures:
-
Linear Molecular Geometry
In a Linear Molecular Geometry structure, atoms are bonded together to form a straight line. Bonding angles are of 180° degrees and as an example is the Carbon Dioxide (CO2) and the Nitric Oxide (NO).
-
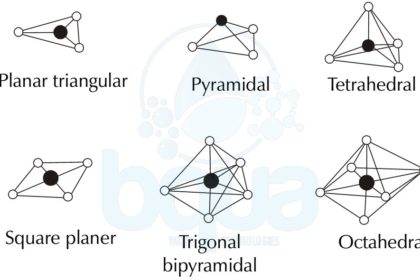
Planar Triangular –Â Trigonal Planar Molecular Geometry
Trigonal Planar Molecualr Geometry is formed when a compound has an atom at the centre attached to three other atoms in an arrangement that looks like a triangle around the central atom. The four atoms are on the same line and flat on a plane.
-
Trigonal Pyramidal Molecular Geometry
Trigonal Pyramidal Molecular Geometry is obviously shaped like a pyramid with a base that looks like a triangle. The Trigonal Pyramidal structure looks like the Tetrahedral Molecular Geometry, pyramidal structures need three dimensions so that they can fully separate electrons. An example on the Trigonal Pyramidal orientation is Ammonia (NH3).
-
Tetrahedral Molecular Geometry
Tetra means four and tetrahedral means basically a solid or pyramid that has four sides. Tetrahedral Molecular Geometry is formed when one central atom has four bonds with four atoms all at once forming a pyramid-like shape with four sides. As per the VSEPR which is the Valence Shell Electron Pair Repulsion theory, the bond angles between the atoms in the tetrahedral orientation are approximately 109.47°. An example of the Tetrahedral Molecular Geometry is methane (CH4).
-
Square Planar Molecular Geometry
The Square Planar Molecular Geometry is formed when a central atom has four bonds and two lone pairs. Xenon Tetrafluoride (XeF4) is an example of the Square Planar structure, it is made up of six equally spaced orbitals arranged at 90° degrees angles. Which forms an octahedral shape. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. And the other four atoms are bonded to the central atom making the molecule a square planar structure.
-
Trigonal Bipyramidal Molecular Geometry
The Trigonal Bipyramidal Molecular Geometry happens when the central atom is connected to five atoms forming five bonds and no lone pairs. Three out of the five bonds are created along the atom equator forming 120° degrees angles while the remaining two are formed on the atom axis. An example on the Trigonal Bipyramidal Molecular Geometry is the Phosphorus Pentachloride (PCl5).
-
Octahedral Molecular Geometry
Octa means eight and Octahedral Molecular Geometry means a pyramid or solid that has eight sides or faces. Octahedral structure has six bonded atoms forming 90° degrees angles between them. An example on the Octahedral Molecular structure is Sulfur Hexafluoride (SF6).
Molecular Shapes and different types of molecular structures